Negative charged subatomic particles located outside the nucleus or in the electron cloud. So pro tars neutrons and luck charms are the three sub atomic particles that make up and Adam let us take carbon for example which is represented.
What Are Atoms And Subatomic Particles And How Do They Work Quora
Proton decides the atomic number of an element because.
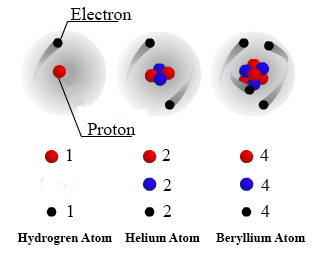
. This number increases as you. The atomic number is used to identify an element and the atomic number is the number of protons in the nuclei of an element. Protons are positive-charged particles found in the atomic nucleus.
B The proton number remains constant through changing the atomic charge energy and isotopes. Were excluding the electrons because that was determined the charge. The Only Subatomic Particle That Does Not Carry An Electric Charge Is The What.
An equal number of electrons and protons are found in the atoms of all elements. No two elements have same number of protons. Neutral or no charged subatomic particle inside the nucleus.
Electron negatively charged subatomic particle 5. Neutron proton and electron. Compound neutral subatomic particle found in the nucleus of the atom 3.
These sub-atomic particles are neutron proton and. Z is the atomic number of the element or subatomic particle and A is the mass number of the atomic nucleus or atomic particle. Every element has its own unique number of protons and therefore atomic number.
The atomic number is consider a unique atom and element identifier. Isotope one of two or more atoms of the same. Describe the associated subatomic particle and how it can be used to identify the atom.
Proton is the subatomic particle which identify the elements identity. Chemistry questions and answers. It is usually considered to be neutrons and protons that hold most of an atoms mass however there is one theoretical subatomic particle that is used to help explain mass called the higgs boson.
Isotope notation is also discussed in this video. A The number of electrons changes in a unique way as the charge on the atom changes. Proton is the subatomic particle which is used to define an element becasue it is unique.
If the number of proton change it changes the identity of element since each element has specific atomic number. In each of the equations X is the unknown atom or subatomic particle formed in the reaction where X is the symbol for a chemical element or subatomic particle. The distinguishing subatomic particle of an atom is itsprotons.
Technically a solitary proton can be considered an atom of an element hydrogen in this case. The mass number is made up of. And Adam as the difference of a tunnel particles.
Atomic number number of protons in the element. The atomic number amu of an element is equal to thenumber of protons it has. A discussion on how each element is defined in terms of the number of its subatomic particles.
There are three different subatomic particles present in the atoms of each element. Colloid two or more elements that have chemically combined 2. An atom is made up of three sub-atomic particles.
Which subatomic particle is used to identify an element. So youre I have three different opportunities that it could occur. Positive charged subatomic particle inside the nucleus.
Goktugg Getty Images. An atom is divided into sub-particles including electrons protons and neutrons. When were talking about a subatomic particle defining an element we should be thinking about the protons and the neutrons.
And theyre not related to the weight in determining mechanism off um element. Distillation positively charged subatomic particle found in the nucleus of the atom 4. First there is the positively charged proton uncharged cone via Lee the negatively charged in the trunk.
Two different elements can not have same atomic numbers. Therefore if you know the number ofprotons in a. The most basic unit of an atom is the proton because the number of protons in an atom determines its identity as an element.
NUMBER protons How you identify an element. Match the following and ill give you the 1. Thompson is credited with the discovery of electrons since he was the first person to accurately calculate the mass and the charge on an electron.
Electrons are negatively charged subatomic particles.
1 8 Subatomic Particles Protons Neutrons And Electrons Chemistry Libretexts


0 Comments